VIDEO TRANSCRIPT: Fuel Cell Energy's direct fuel cells generate electricity by combining fuel and air in a clean and efficient process without combustion. In-fact, as long as you supply fuel, it's like a battery that never loses its charge. A fuel such as pipeline natural gas or renewable fuel like waste water treatment gas is fed to the anode and air is fed to the cathode in a process called reforming. Hydrogen is extracted from the fuel and is electro chemically reacted with the oxygen in the air to produce electricity, heat and water. Fuel Cell Energy's direct fuel cell technology is unique among fuel cells in that this hydrogen process takes place inside the fuel cell. No external reforming is required. When multiple fuel cells are stacked on top of each other, they can produce hundreds of kilowatts of power for use in a variety of settings. The world is looking for innovative energy to power the future and fuel cell energy is delivery today.
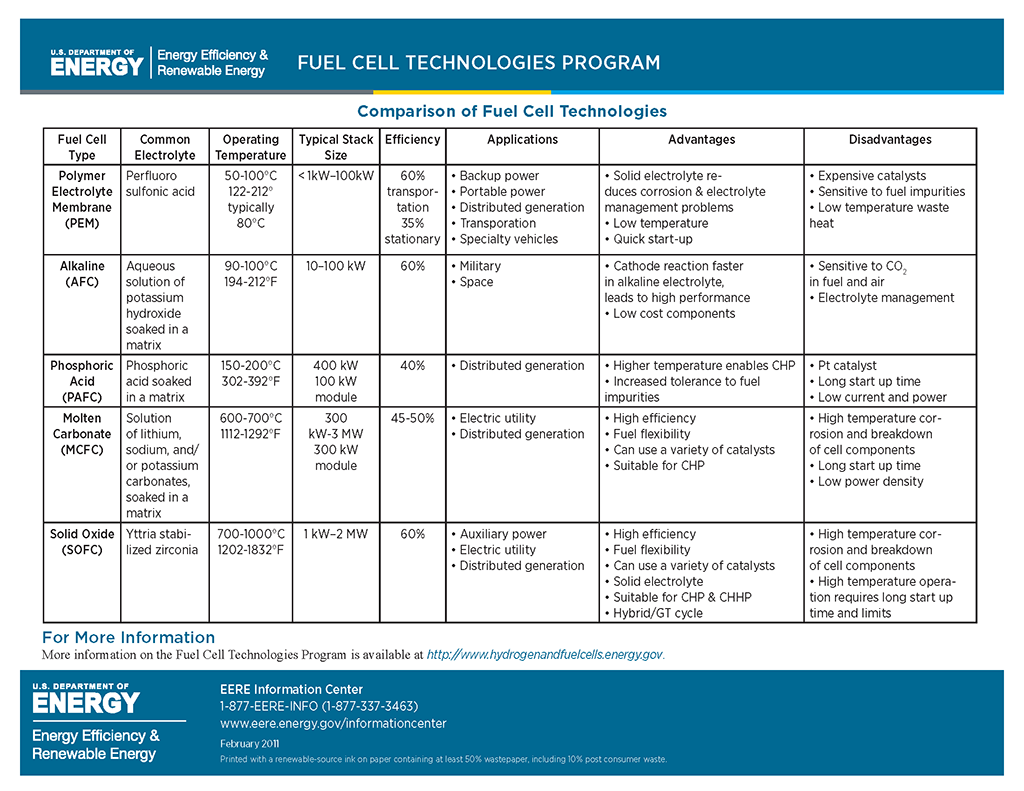
Molten Carbonate Fuel Cell
A molten carbonate fuel cell is made up of an electrolyte member sandwiched between fuel and oxidant electrodes. Typically, a fossil fuel or biogas from which hydrogen is extracted is used for most common applications. The oxidant is typically plain air. The fuel is fed to the anode and air is fed to the cathode in a process called reforming. The fuel is oxidized at the “anode electrode”, releasing electrons that move to the “cathode electrode” via the external circuit. These electrons meet the hydrogen and push charged ions across the electrolyte. The charged ions (positively or negatively charged) move across the ion conducting electrolyte member, completing the electrical circuit.
This electrochemical process requires very few moving parts, typically limited to air blowers and fuel/water pumps. Molten carbonate fuel cell power plants can utilize many fuel sources, including natural gas, industrial and municipal wastewater treatment gas, propane, and coal gas. These kinds of fuel cells use an electrolyte made up of a carbonate salt that operates at a temperature exceeding 1000°F (540°C). By using high temperature molten carbonate, hydrogen conversion from the fuel source is improved, resulting in higher efficiencies than those achieved with low-temperature fuel cells.
The heat generated as a result of the electrochemical process in a molten carbonate fuel cell can also be used for secondary purposes in what is known as a combined heat and power (CHP) process. By using the waste heat, the efficiency of the fuel cell can increase to approximately 47-80%, compared to only 25-35% for a turbine/engine system.